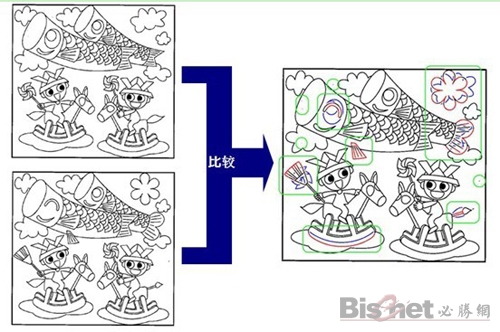
Fuji Xerox new technology-drawing difference detection system
Drawing difference detection system is a detection technology that can automatically find differences in drawings. The drawings before and after the modification are directly scanned by the Fuji Xerox digital multi-function machine or computer network and transmitted to the drawing difference detection system. After the two sheets of drawings are overlapped, the system finds the difference images through light transmission, and prints them in different colors or sends them in the form of electronic files. At the same time, after the difference detection is completed, the system will automatically send a job completion notification email.
The Outport Stopper For I.V Fluid Bag is based on the polycarbonate joint formed by injection molding process, comprising of the injectionâ€purpose halogenated butyl rubber stopper and aluminumâ€plastic composite cap. Among which, the material for polycarbonate joint is the imported medicalâ€level polycarbonate, complying with the FDA rules; the aluminumâ€plastic composite cap is produced by using the imported topâ€quality aluminum strip; the injectionâ€purpose halogenated butyl rubber stopper can be chosen to use the wellâ€known domestic product or imported product on the request of user.
The infusion stopper is produced in the clean plant, with the partial part reaching to Class 100 under the Class 10,000 background. The main working procedures, such as the injection molding and the cleaning, riveting, inspection, wash, assembly and etc., are designed and processed as per the cleanliness requirements at the partial level of Class 100, which passes the GMP onâ€site certification and complies with the cleanliness requirements of high capacity injection medicine.
The main production equipments, test and inspection instruments of infusion stopper have already reached to the international level and are advanced at home. Meanwhile, the full production process is conducted the strict control and management, and making product free of plasticizing agent, loading agent and lubricating agent, and free of absorption or transfer when contacting with liquid medicine, neither the pyrogen and soluble matter, thence such equipment may ensure the product quality.
The outport stopper for I.V fluid bag is mainly used for doubleâ€hose nonâ€PVC software bag.
Outport Stopper For I.V Fluid Bag Stopper For I.V Bag,Stopper For Plastic Infusion Bag,I.V Fluid Bag Stopper,I.V Fluid Bag Outport Stopper YANGZHONG HONGYUN BOTTLE CAPS MAKING FACTORY , https://www.hongyunyz.com